___ Described the Location of Electrons Using 4 Quantum Numbers
The carbon C has 6 electrons it s electronic configuration is. One electronn 4 l 2 ml2 m l 2 and ms -12.
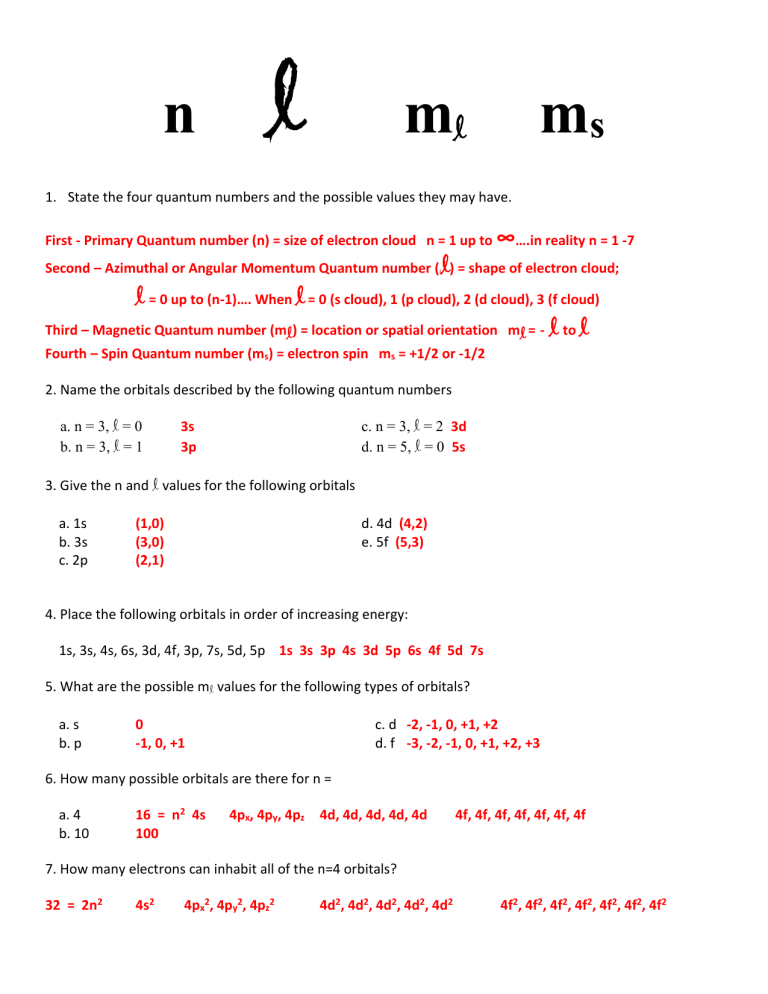
Quantum Numbers Introduction To Chemistry
So the fourth electron is in the 2s orbital the four quantum to describe it would be.

. 5 How many orbitals can the. The azimuthal quantum number also known as the orbital angular momentum quantum number. PRINCIPAL QUANTUM NUMBER n - Represents the main energy level or shell occupied by an electron.
The four quantum numbers that can be employed for this purpose include. True - they will always have at least one different because they cannot both spin the same direction. Quantum Numbers Write the four quantum numbers which describe the location of the highest energy electron of the following.
The number and letter pairs in an electron configuration represent two of the electrons four quantum numbers. Each orbital may hold up to two electrons with opposite spin directions. It is always a positive integer that is n 1 2 3.
The Four Quantum Numbers. The principal quantum number largely determines the energy of an electron. But one may also use unrestricted orbitals where alpha and beta electrons do not share the same spatial part.
Electrons in the same atom that have the same principal quantum number are said to occupy an electron shell of the atom. Ni 28 -12 28 electron 16 e o mi ms 3. The quantum numbers are parameters that describe the distribution of electrons in the atom and therefore its fundamental nature.
1 How Many Electrons In An Atom Can Have The Quantum Numbers N 4 L 2. Here it is obviously necessary to include the spin quantum number. N principle quantum number - energy shell l angular momentumaz.
This video provides 3 example practic. The quantum numbers for electron 1 hydrogen are. How Many Electrons In An Atom Could Have These Sets Of Quantum Numbers N3Eighteen electronsHow many electrons can the following set of quantum numbers n 3two electronsSince each orbital can hold a maximum of two electrons the number of electrons that can share the two quantum number n3 and ml.
N 72 111 et 7 elections 112 ins 2. To completely describe an electron in an atom four quantum numbers are needed. 100 1 rating Transcribed image text.
Quantum numbers are important because they. Electrons are not really spinning in a physical sense this is just a representation of the idea that there are two possible values for the spin quantum number. This is called the spin quantum number s because electrons behave as if they were spinning in either a clockwise or counterclockwise fashion.
The principal quantum number can be any nonzero positive integer. The combination of all quantum numbers of all electrons in an atom is described by a wave function that complies with the Schrödinger equation. So the above set of the quantum numbers describes only one electron.
Ms spin number - tells us if the electron is spin up or spin down. The principal quantum number n the angular momentum quantum number l the magnetic quantum number m_l. 2 How many electrons in an atom may have the following quantum numbers n 4 L 2.
The first quantum number is called the principal quantum numbern. What Do Quantum Numbers Describe. 3 How many electrons can have the following quantum numbers n 4 L 2 ml 1 and MS 1 2.
The value of n ranges from 1 to the shell containing the outermost electron of that atom. 4 How many electrons in an atom can have the quantum number set N 4 L 3. How many electrons can be present in each orbital.
The first quantum number describes the electron shell or energy level of an atom. According to the Pauli Exclusion Principle no two electrons can share the same combination of four quantum numbers. S d p f.
The principal quantum number often denoted by the symbol n. According to Paulis Exclusion Principle no two-electron present in an atom can have the same set of values for four quantum numbers. You have values for three our of the four quantum numbers that we use to describe the location and the spin of an electron inside an atom.
- Ill recommend this video to help. These quantum numbers tell us more information about the properties of electrons and. Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one.
Spin Quantum Number Individual electrons within an orbital has a property represented by the spin quantum number. Each electron in an atom has a unique set of quantum numbers. Angle quantum number - subshell ml magnetic quantum number - particular orbital within subshell ms magnetic spin number - spin direction-They relate to the pauli exclusion principle in that these 4 numbers are different fro every electron of an atom.
TF No two electrons in the same atom have the same 4 quantum numbers. The location of electrons is described by an electron configurationThe electron configuration gives enough information to know where the element is on the. When an electron is assigned to.
This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence. One of the electrons in an orbital is arbitrarily assigned an s quantum number of 12 the other is assigned an s quantum number of -12. 1 2 3 4.
There is a lot of info youll need to understand. กร ne M2 Ms Conceptual Exercise 9. There are actually 5 quantum numbers that specify an orbital.
The movement and the trajectory followed by an electron in an atom can be described with the help of quantum numbers. The electrons identified by quantum numbers n and l i n 4 l 1 ii n 4 l 0 iii n 3 l 2 iv n 3 l 1 can be placed in order of increasing order of increasing energy from the lowest to highest as. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s.

Quantum Numbers Video Quantum Physics Khan Academy

No comments for "___ Described the Location of Electrons Using 4 Quantum Numbers"
Post a Comment